임상 표본으로 검증 : 코로나-19 진단 개선 연구
- 저자Jasper Fuk-Woo Chan, Cyril Chik-Yan Yip,Kelvin Kai-Wang To, Tommy Hing-Cheung Tang,외 저
- 출판사아진
- 출판일2020-07-12
- 등록일2020-12-21
- SNS공유


- 파일포맷PDF
- 파일크기94MB
- 공급사YES24
-
지원기기
PC
PHONE
TABLET
프로그램 수동설치
전자책 프로그램 수동설치 안내
아이폰, 아이패드, 안드로이드폰, 태블릿,
보유 1, 대출 0,
예약 0, 누적대출 6, 누적예약 0
책소개
On 31 December 2019, the World Health Organization was informed of a cluster ofcases of pneumonia of unknown etiology in Wuhan, China. Subsequent
investigations identified a novel coronavirus, now named severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2), from the affected patients. Highly
sensitive and specific laboratory diagnostics are important for controlling the
rapidly evolving SARS-CoV-2-associated coronavirus disease 2019 (COVID-19)
epidemic. In this study, we developed and compared the performance of three
novel real-time reverse transcription-PCR (RT-PCR) assays targeting the
RNA-dependent RNA polymerase (RdRp)/helicase (Hel), spike (S), and nucleocapsid
(N) genes of SARS-CoV-2 with that of the reported RdRp-P2 assay, which is used
in 30 European laboratories. Among the three novel assays, the
COVID-19-RdRp/Hel assay had the lowest limit of detection in vitro (1.8 50%
tissue culture infective doses [TCID50]/ml with genomic RNA and 11.2 RNA
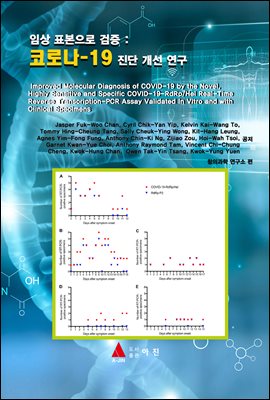
copies/reaction with in vitro RNA transcripts). Among 273 specimens from 15
patients with laboratory-confirmed COVID-19 in Hong Kong, 77 (28.2%) were
positive by both the COVID-19-RdRp/Hel and RdRp-P2 assays. The COVID-19-
RdRp/Hel assay was positive for an additional 42 RdRp-P2-negative specimens
(119/ 273 [43.6%] versus 77/273 [28.2%]; P 0.001), including 29/120 (24.2%)
respiratory tract specimens and 13/153 (8.5%) non-respiratory tract specimens. The
mean viral load of these specimens was 3.21104 RNA copies/ml (range, 2.21102 to
4.71105 RNA copies/ml). The COVID-19-RdRp/Hel assay did not cross-react with
other human-pathogenic coronaviruses and respiratory pathogens in cell culture
and clinical specimens, whereas the RdRp-P2 assay cross-reacted with SARS-CoV
in cell culture. The highly sensitive and specific COVID-19-RdRp/Hel assay may
help to improve the laboratory diagnosis of COVID-19.
목차
제 1편 코로나바이러스 정의1. 코로나바이러스감염증-19(Covid-19) 정보 7
2. 코로나바이러스 분류 및 특성 9
3. 코로나바이러스 전자현미경 형태 11
4. 코로나바이러스 구조 (Covid-19 Organization) 13
5. 코로나19: 환경에 지속적인 영향을 미칠까? 19
6. 치료법(Therapeutical Method) 22
제 2편 연구논문
Improved Molecular Diagnosis of COVID-19 by the Novel, Highly
Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse
Transcription-PCR Assay Validated In Vitro and with Clinical
Specimens
1. ABSTRACT 23
2. MATERIALS AND METHODS 24
3. RESULTS 25
4. DISCUSSION 29
5. SUPPLEMENTAL MATERIAL 31
6. ACKNOWLEDGMENTS 31
7. REFERENCES 31